Submitting a Monograph
Guidelines for Clinical Verification
Approved July 2020
7. Data Analysis
Clinical data is acquired and analyzed in a fundamentally different manner than data from homeopathic provings. In homeopathic provings, the homeopathic clinical picture of the IHMP may be unknown. Subjects are volunteers randomly selected from the general population who may or may not demonstrate sensitivity to the IHMP (see Figure 2 below). In acquiring clinical data, on the other hand, inclusion criteria derived from the drug discovery data are used to define an approximate homeopathic clinical picture for which the IHMP is likely to be effective. Using criteria derived from drug discovery, subjects who have a higher degree of coherence with respect to their clinical characteristics will be selected. Such subjects should demonstrate greater uniformity in how they respond to the IHMP. Successful treatment with the IHMP helps clarify the homeopathic clinical picture of those patients (or subjects in an experimental trial) more likely to respond to the IHMP.
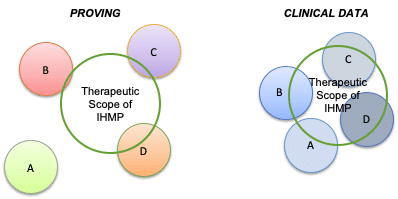
Figure 2: Therapeutic scope of the IHMP is the same in both Provings and in Clinical Use. However, the subjects who receive the IHMP (A-D) are randomly selected from the general population in the Proving, but selected based upon specific indications during acquisition of clinical data. In both situations, some subjects are more closely individualized to the IHMP, but in the clinical data group, the similarity should be higher among subjects who demonstrate a positive outcome to treatment. By selecting a subset of subjects who respond to the IHMP treatment, the homeopathic clinical picture for the IHMP may be further distilled.
The HPCUS will evaluate clinical data submitted by a monograph sponsor in a manner consistent with the fundamental tenets of homeopathic medicine and with an aim to minimize bias. Any of the following three types of data may be submitted for consideration:
1. Case reports / Case series
2. Observational studies
3. Experimental trials
While the method of evaluation will vary according to type of data, the overall assessment goal remains the same: the clinical data must demonstrate a sufficient number of assessable outcomes to provide a homeopathic clinical picture (indications) for the IHMP. This homeopathic clinical picture developed from the acceptable drug discovery and clinical data must support a reasonable degree of confidence in successfully selecting the IHMP for treatment purposes. The emphasis is on obtaining a sufficient (rather than a conclusive) amount of evidence, as the clinical experience gathered over time should enhance the overall clarity of homeopathic clinical picture and extent of therapeutic usefulness of the IHMP.
- General Requirements
Method of data analysis shall be matched to the type of data acquired for monograph submission.
Clinical data submitted with comparative data (i.e., historic norms or control results) shall be accompanied by relevant statistical analysis.
Reasons for selection and acquisition of any comparative data shall be reported and justified.
- Case Reports / Case Series
Case reports shall be submitted and evaluated with particular attention to the following aspects:
a) Clinical case reporting guideline requirements, including:
i. The CARE guideline for reporting clinical cases
ii. The HOM-CASE CARE guideline extension for homeopathic case reports
b) Attribution of changes in cases to the IHMP:
iii. Potential for bias with regard to the attribution of observed changes to the IHMP
iv. Ratings based on the HPCUS Case Outcome Assessment Tool (see Section 4.4.7).
c) Number of cases with acceptable reporting and methodology that have sufficient outcome data (see Figure 3 below)
d) Characteristic features of the emergent homeopathic clinical picture of IHMP
Figure 3: Case / Case Series Analysis
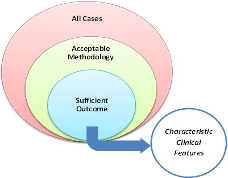
Steps. In the review process by the PRC, all submitted cases for monograph evaluation will be reviewed. Those with both acceptable methodology and sufficient outcome to demonstrate at least a probable effect of the treatment will be evaluated for characteristic features. Quantitative and qualitative evaluation with regards to these cases will be used to determine if the IHMP is associated with a sufficient homeopathic clinical picture.
Each submitted case shall include a detailed list of symptoms and signs considered to be relevant to treatment with the IHMP. Symptoms and signs shall be evaluated according to the following criteria:
a) Severity
b) Degree of importance to the subject
c) Assessed degree of importance to the clinician
d) Magnitude of change during the treatment course (along with direction of change, whether improved or worsened)
e) Time period in which change was observed
7.2.1. Step One: Outcome Assessment
The first level of analysis is an assessment of overall outcome for the case as well as for changes in individual symptoms.
Outcome assessment for each submitted case will include the following:
a) Subjective global health assessment ratings from the case subject
b) Global health change assessment must be included
i. Measures of time period showing
pre-treatment and post-treatment points of assessment shall be included
i. Use of validated Patient Reported Outcome Measurements (PROM) scale (i.e., PROMIS® or SF36) for global health assessment is recommended
c) Individual symptom assessment:
i. Subjective symptom change assessment must be included
ii. Objective symptom / findings change assessment shall include:
• Any observed changes (including examination, physical testing, laboratory results, and other tests such as radiological examinations) shall be included
Validated symptom scales for individual symptoms are recommended if cases are selected for one specific diagnostic category
• Clinician-observed changes in individual symptoms shall be included as objective findings
• Visual analogue scale or other similar instrument for change in individual symptoms is recommended
• Estimate regarding the plausibility of time course for symptom change is recommended
7.2.2. Step Two: Assessment for Clinical Features
Cases with both acceptable methodology and sufficient outcome to demonstrate a highly probable effect of the treatment shall be used as a basis for identifying clinical features. Characteristic clinical features, if present, shall be identified and assessed as a subset of particular interest.
This analysis shall include the following:
d) Unexpectedly improved symptoms or objective findings
e) Characteristic and/or highly characteristic symptoms
f) Commonly occurring modalities
g) Frequently occurring symptoms or groups of symptoms related by “red-line” analysis
h) Shared types of pathology or tendencies
i) Reporting of frequently observed symptoms that have not changed and are unlikely to change with any treatment is recommended, but must not form a substantial portion of this analysis
j) Inclusion of quantitative analysis using likelihood ratios or similar instruments is recommended to further support the prognostic value of particular clinical features as indicators for successful treatment with the IHMP
7.2.3. Step 3: Assessment for Similarity of Cases
Case coherence supports characterization of the homeopathic clinical picture of the IHMP. However, assessment for coherence of cases is not required for monograph submission.
It is recommended that an additional level of analysis be conducted to identify clinically similar subjects who demonstrated a positive response to treatment with the IHMP. If conducted, this analysis could include:
a) Report of the shared clinical features among subjects who demonstrated an improvement upon outcome assessment
b) Categorization of signs and symptoms from cases using repertory style rubrics or other categories that adequately capture the clinically important and relevant information from the individual subject cases
c) Comparative symptom and sign analysis across individual subjects that may include the following parameters:
i. Shared and unshared signs / symptoms present in the subject case
ii. Shared and unshared signs / symptoms that were clinically improved at time of outcome evaluation
7.2.4. Step 4: Assessment for Homeopathic Clinical Picture
A final level of analysis shall be conducted to assess for an emergent homeopathic clinical picture related to the IHMP.
This analysis shall involve a qualitative synthesis of the most homeopathically relevant and reliable data on the IHMP with particular attention to the following:
a) Characteristic clinical features
b) Recurring clinical features across multiple cases
c) Cases that demonstrate improvement of similar types of clinical features
d) Common threads or red-line symptoms that demonstrate coherence within and across subjects
e) Emergent indications for the IHMP that arise from evaluation of these listed types of data
- Observational Studies
Outcome assessment shall make use of validated outcome measures whenever possible.
Assessment of the likelihood of causal attribution of the observed changes in subjects due to the IHMP shall take place. Any reported changes must demonstrate a reasonable level of confidence that the change was due to the IHMP.
A comparison calculation such as the Likelihood Ratio is recommended to demonstrate the relationship of any variables of interest to the IHMP.
Symptoms associated with the IHMP in likelihood calculations are based on the treatment response -as a whole- to the IHMP. It is recommended that single symptoms or signs that did not, or cannot, improve in response to treatment with the IHMP still be included as part of the homeopathic clinical picture of (prognostic indicators for) the IHMP when significant.
The criteria used to determine treatment response shall be clearly defined and transparent. “Responders” must experience a clinically significant improvement which is attributable to the IHMP administration.
- Experimental Trials
The analysis strategy will vary depending on the type of trial.
Homeopathic symptoms collected at baseline (without being used in the individualization of treatment) can be investigated post-hoc by analyzing for symptom by IHMP treatment interaction (effect-modification) using the appropriate statistical test.
Primary endpoint analysis shall be conducted and reported.
Additional analysis supporting the development and clarification of a homeopathic clinical picture shall be included.
- Pharmacopeia Quality Review Procedure
The Pharmacopeia Revision Committee (PRC) and Monograph Review Committee (MRC) of the HPCUS will perform independent but linked reviews of any sponsor submitted data for monograph purposes. The following guidance is provided as a reference for those working committees.
MRC will conduct a review for monograph purposes based on the HPCUS Technical Submission Guidelines for Monograph Review.
PRC will evaluate all submitted data for monograph purposes using both the HPCUS Proving Guidelines and these Clinical Data Guidelines for reference to the requirements and recommendations for monograph sponsors.
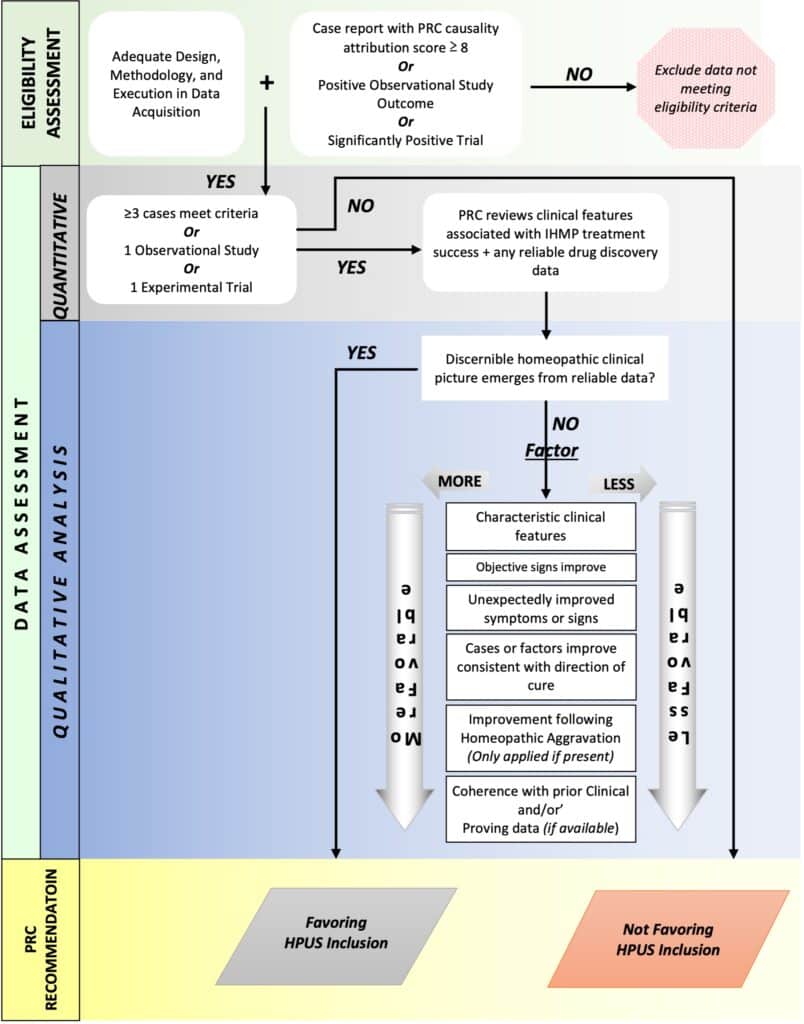
PRC members shall pay particular attention to the following elements during the monograph review (as summarized in Flowchart 2 PRC Clinical Data Quality Review Template that follows at the end of this section:
a) Content and reliability of any existing background data including toxicology reports, proving data, traditional use data, and historical use information of the IHMP
b) Adequacy of design, methodology, and execution in the acquisition of new data on the IHMP
c) Type and quantity of new data
d) Adherence to homeopathic principles in the gathering, evaluation, and reporting of new data
e) Appropriateness of qualitative and/or quantitative methods used to assess outcomes for use of the IHMP
f) Potential for bias when collecting new data
g) Exclusion of any data likely to be biased
h) Presence or absence of a discernible homeopathic clinical picture resulting from all data provided
i) Sufficiency of data to support the emergent homeopathic clinical picture in terms of both quality and quantity of data. Additional data may be requested
Independently, MRC and PRC shall each come to a decision of the suitability of the IHMP for inclusion in the HPUS.
Report of MRC and PRC recommendations shall be forwarded to the Board of Directors through the Editor as either “Favoring inclusion” or “Not favoring inclusion.”
Flowchart 2: PRC Clinical Data Quality Review template
i) Gagnier JJ, Kienle G, Altman DG, et al. ; and the CARE group. The CARE guidelines: consensus-based clinical case report guideline development. Glob Adv Health Med. 2013;2(5):38–43. Doi: 10.7453/gahmj.2013.008. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3833570/. Published online 2013 Sep 1. Accessed September 10, 2020.
