Official Monograph Review Procedure
Monograph Submission and Review Procedure
The HPCUS evaluates New Monograph Proposals in the form of a dossier submitted by Sponsors for consideration for inclusion in the Homoeopathic Pharmacopoeia of the United States (HPUS). In addition, the HPCUS may periodically review extant Monographs when new information is made available or when concerns regarding clarity or accuracy are raised.
New Monograph Evaluation
Monographs may be submitted to HPCUS by external Sponsors or by HPCUS itself. The monograph should be prepared in a format and with supporting documentation and safety data according to the guideline documents in the HPUS. New Monographs will be evaluated according to current HPCUS Guidelines on Monograph Review and HPCUS Criteria for Eligibility for Inclusion in the HPUS at the time of submission. All New Monographs must receive HPCUS Board Approval prior to entry into the HPUS.
Procedure in Brief
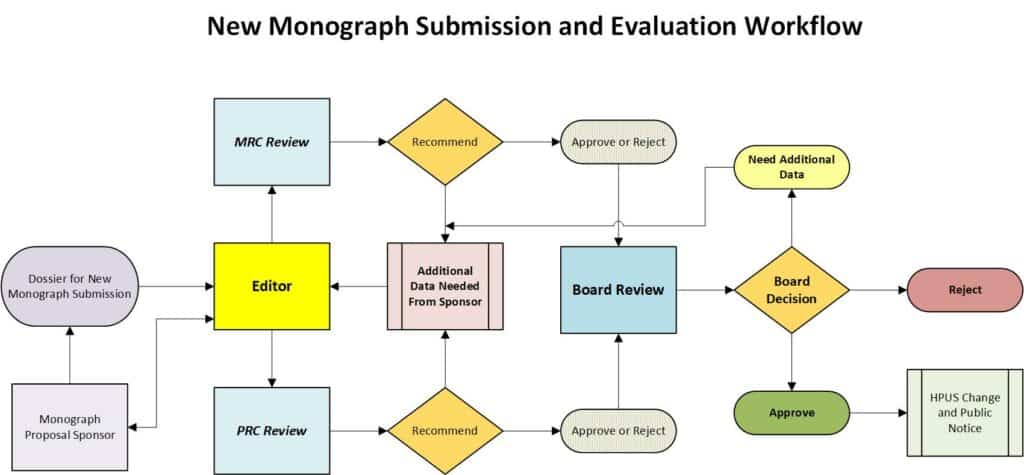
- Any New Monograph Proposal shall be submitted to the Editor for preliminary review and comment. A complete Monograph Submission includes both a Main Monograph document and a Quality Control Specifications document along with all supporting documentation. Submissions must be accompanied by the appropriate Monograph Submission fee.
- The Editor shall forward the Monograph Submission to the MRC and PRC committees for review and recommendation.
- The MRC shall review the submission for Monograph consideration according to HPCUS Guidelines to Technical Information Requirements for Monograph Review. Concurrently, the PRC shall review the submission for Monograph consideration according to HPCUS Proving Guidelines, the HPCUS Clinical Data Guidelines, or both.
- Upon completion of reviews, each respective committee shall make a recommendation regarding the submission for New Monograph consideration and forward that recommendation to the HPCUS Board according to one of the following categories: Recommend Approval, Recommend Additional Information Required, Recommend Rejection.
- The Board will review the recommendations as well as the monograph submission and can decline, return the Monograph to the Sponsor for additional data, or approve the Monograph for publications
- Approved new HPUS Monographs shall be published as official and public notice shall be provided per the HPCUS Change Control Policy.
A Schematic for New Monograph Review is included below for reference.
Revisions to existing HPUS Monographs shall be published and referred for either public notice or public comment in accordance with the HPCUS Change Control Policy.
Extant Monograph Review
Monographs that have been previously approved may be re-reviewed at the request of an HPCUS committee or working member or by an external entity (such as a manufacturer or regulatory authority) upon approval for the review by the HPCUS Board.
Procedure in Brief
- The Board may direct specific committee(s) or specialized panels of committees to conduct a review according to the specific concerns regarding the Monograph. (i.e., for newly recognized toxicological concerns, the Toxicology and Safety Committee may primarily conduct a review). Relevant external subject matter experts may be utilized, if required.
- On completion of committee review, the committee shall make a specific recommendation for action. Non-substantive changes may be implemented by the committee. Recommendations for any substantive changes to monographs shall be forwarded to the Board for review.
- The Board can accept, decline, or return the recommendations to the same or to another committee for additional review or clarification.
- The Board will determine if the change to the Monograph is substantial enough to warrant a period of public comment, and for what duration such a period remains open.
- Following any public comment period, the change shall be published as official.
The above is a synopsis of the procedure. For more details, interested parties can request a copy of the HPCUS Monograph Evaluation and Review Policy. The details in the full policy shall guide the actual review process to be conducted by the HPCUS.
